Causes: Carbon dioxide CO2
Image: Patrick Hendry
Causes
- A brief history of climate change: who knew what, when
- What causes climate change?
- Would the climate be warming without humans?
- Is it just a cycle? (Earth’s wobbly orbit)
- Sunspots & solar activity
- Land use: agriculture & cities
- Volcanoes
- Ocean currents
- Black carbon & ash
- Hydrogen
- Greenhouse gases & how they work
- – Carbon dioxide & the carbon cycle
- – Methane: biogenic (mostly cows) & ‘natural’ gas
- – Nitrous oxide
- – Clouds & water vapour
- – Ozone
- – Man-made industrial chemicals
- – Aerosol pollution
- How to start an Ice Age!
- What’s in a name?
Other sections
Home > Climate wiki > What causes climate change? > Greenhouse gases > Carbon dioxide
Carbon dioxide (CO2) and the carbon cycle
Summary
“The effect of increasing the concentration of atmospheric carbon dioxide on global average surface air temperature might be expected to be constant. However, doubling the atmospheric CO2 concentration increases the impact of any given increase in CO2 by about 25%, owing to changes induced in the climatological base state. The more anthropogenic CO2 emissions raise the atmospheric CO2 concentration, the more serious the consequences will be.” — Science, 30 Nov. 2023
- Carbon is in the rock and soil, the oceans, all living things, and the atmosphere. How it cycles between them is called the carbon cycle (Figs. 2 & 3 in the tabs below).
- Carbon is a powerful climate stabiliser. It acts like a control nob on the planet’s thermostat. When combined with oxygen to become the gas CO2 in the atmosphere, it keeps Earth warm. This happens as living things take carbon from the atmosphere and ocean and use it to grow bones and teeth and shells. When they die, some carbon is locked underground as calcium carbonate and turned into limestone (Fig. 3).
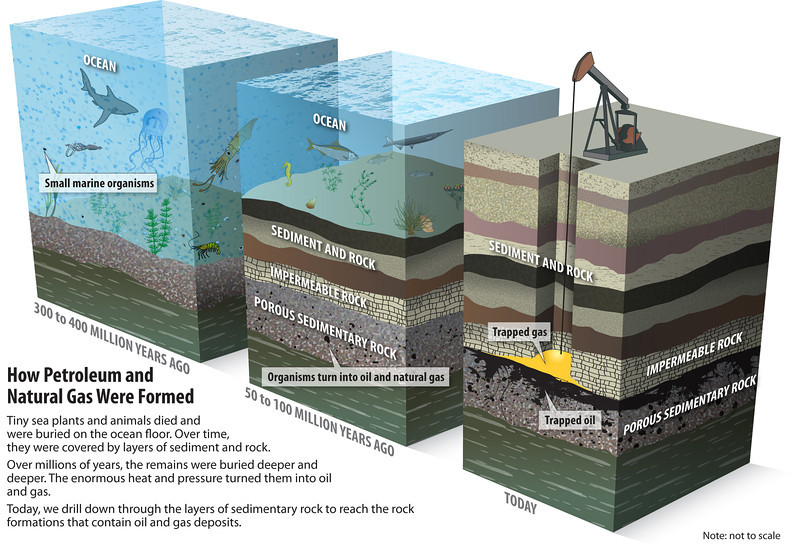
- Other processes turned the dead plants and animals into coal and oil (Figs. 2, 6 & 7 in the tabs below). When this happens over millions of years, the amount of carbon underground goes up, while it goes down in the atmosphere so Earth cools. At other times and over equally long periods volcanoes (and the odd comet) have released enough CO2 into the atmosphere to warm Earth.
- This natural cycle has slowly moved the climate between warm ‘hothouse’ and cold ‘icehouse’ states since the planet formed ~4.6 billion years ago.
- In less than 200 years humans have moved more carbon from the ground into the atmosphere than it took natural process hundreds of millions of years to do. We know this because carbon comes in three forms (isotopes): 12C, 13C and 14C. Their relative ratio in the atmosphere is a chemical fingerprint (see the volcano tab below) that points squarely at humans (Kiwi kids helped measure this).
- Around half the excess carbon dioxide that we’ve emitted into the atmosphere has been absorbed by the superhero of climate change: our oceans. However, this is making them more acidic.
Causes
- A brief history of climate change: who knew what, when
- What causes climate change?
- Would the climate be warming without humans?
- Is it just a cycle? (Earth’s wobbly orbit)
- Sunspots & solar activity
- Land use: agriculture & cities
- Volcanoes
- Ocean currents
- Black carbon & ash
- Hydrogen
- Greenhouse gases & how they work
- – Carbon dioxide & the carbon cycle
- – Methane: biogenic (mostly cows) & ‘natural’ gas
- – Nitrous oxide
- – Clouds & water vapour
- – Ozone
- – Man-made industrial chemicals
- – Aerosol pollution
- How to start an Ice Age!
- What’s in a name?
Other sections
Home > Climate wiki > What causes climate change? > Greenhouse gases > Carbon dioxide
Carbon dioxide (CO2) and the carbon cycle
Summary
“The effect of
increasing the concentration of atmospheric carbon dioxide on global
average surface air temperature might be expected to be constant.
However, doubling the atmospheric CO2 concentration increases the impact of any given increase in CO2 by about 25%, owing to changes induced in the climatological base state. The more anthropogenic CO2 emissions raise the atmospheric CO2 concentration, the more serious the consequences will be.” — Science, 30 Nov. 2023
- Carbon is in the rock and soil, the oceans, all living things, and the atmosphere. How it cycles between them is called the carbon cycle (Figs. 2 & 3 in the tabs below).
- Carbon is a powerful climate stabiliser. It acts like a control nob on the planet’s thermostat. When combined with oxygen to become the gas CO2 in the atmosphere, it keeps Earth warm. This happens as living things take carbon from the atmosphere and ocean and use it to grow bones and teeth and shells. When they die, some carbon is locked underground as calcium carbonate and turned into limestone (Fig. 3).
- Other processes turned the dead plants and animals into coal and oil (Figs. 2, 6 & 7 in the tabs below). When this happens over millions of years, the amount of carbon underground goes up, while it goes down in the atmosphere so Earth cools. At other times and over equally long periods volcanoes (and the odd comet) have released enough CO2 into the atmosphere to warm Earth.
- This natural cycle has slowly moved the climate between warm ‘hothouse’ and cold ‘icehouse’ states since the planet formed ~4.6 billion years ago.
- In less than 200 years humans have moved more carbon from the ground into the atmosphere than it took natural process hundreds of millions of years to do. We know this because carbon comes in three forms (isotopes): 12C, 13C and 14C. Their relative ratio in the atmosphere is a chemical fingerprint (see the volcano tab below) that points squarely at humans (Kiwi kids helped measure this).
- Around half the excess carbon dioxide that we’ve emitted into the atmosphere has been absorbed by the superhero of climate change: our oceans. However, this is making them more acidic.
Instructions for interactive graphs (Credit: The 2°Institute.)
- Mouse over anywhere on the graphs to see the changes over the last thousand years.
- To see time periods of your choice, hold your mouse button down on one section then drag the mouse across a few years, then release it.
- To see how this compares to the past 800,000 years, click on the ‘time’ icon on the top left.
- To return the graphs to their original position, double-click the time icon.
- The annual ups and downs in the graph are because plants accumulate carbon in the spring and summer and release some back to the air in autumn and winter. As the northern hemisphere has more land and more plants, carbon dioxide levels go up in winter because plants become less productive. Annual measurements of carbon dioxide are an average of these ups and downs.
- Instructions for interactive graphs (Credit: The 2°Institute.)
- Mouse over anywhere on the graphs to see the changes over the last thousand years.
- To see time periods of your choice, hold your mouse button down on one section then drag the mouse across a few years, then release it.
- To see how this compares to the past 800,000 years, click on the ‘time’ icon on the top left.
- To return the graphs to their original position, double-click the time icon.
- The annual ups and downs in the graph are because plants accumulate carbon in the spring and summer and release some back to the air in autumn and winter. As the northern hemisphere has more land and more
plants, carbon dioxide levels go up in winter because plants become less productive. Annual measurements of carbon dioxide are an average of these ups and downs.
-
Plants need CO2 to grow, releasing O2 (oxygen) as a waste product. Animals and people need O2, and they breathe out CO2 as a waste product. Because CO2 is a greenhouse gas it regulates Earth’s temperature. When there’s less CO2 in the atmosphere, more heat from the sun escapes from the atmosphere and Earth cools. When there’s more CO2 in the atmosphere, the opposite happens: Earth warms.
How the carbon moves around the planet, from deep in the oceans, through plants, animals, and the atmosphere is called the carbon cycle (See Fig. 2 the ‘fossil fuel’ part and Fig. 3 the ‘limestone’ part, in the tabs below).
There are four main stages, however, there is no ‘start’ or ‘stop’ point, as the cycle is continuous:
Photosynthesis
Plants on land and in the ocean draw in CO2 (carbon + oxygen) from the atmosphere or seawater and use solar energy + H2O (water) to make carbohydrates (C6H12O6), which they use to grow. They release some CO2 along with an unwanted bi-product, (O2) into water and air, which animals and people use in respiration.Respiration
Animals (including people) take in the O2 made by plants, and exhale CO2, which goes into the atmosphere. Plants use some of this for photosynthesis. However, during respiration plants also release about half the CO2 that they took up. As temperatures increase, the amount of CO2 they release also increases (see the tab ‘Isn’t more CO2 good for plants?)Decomposition
When plants and animals die, if they’re not eaten, they decompose in the soil or fall to the bottom of the ocean (about 18% of our bodies are carbon, and, like our bones and teeth, coral and the shells of marine animals is made of calcium carbonate). Some gasses from decomposition, including CO2, escape into the atmosphere, but depending on where these plants and animals die and how quickly they are buried, quite a bit of the carbon is locked away underground. Over tens or hundreds of millions of years, it can become oil, coal, or limestone.“The world’s soils contain more carbon than terrestrial vegetation and the atmosphere combined.” – Nature GeoscienceCombustion
When fossil fuels are burned for energy (combusted), oxygen (O2) is used and CO2 is released. The more combustion occurs, the more O2 is taken out of the atmosphere. Humans are burning staggering quantities of fossil fuels for energy, so equally staggering amounts of CO2 is being released into the atmosphere, while O2 levels are falling. (Forests are also being burned, but the amount from fossil fuels for energy is orders of magnitude greater.)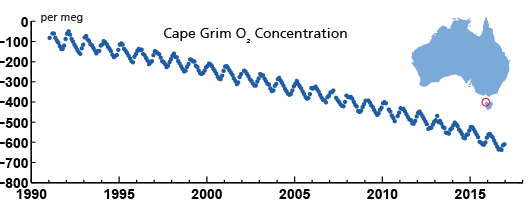
The graph above shows the decline in oxygen measured at Cape Grim, Tasmania. The location was selected to measure Earth’s atmosphere in 1976 because winds from Antarctica and the Indian Ocean hit no significant land masses in the way. The ups and downs in the graph are because there is a natural annual summer/winter ‘cycle’ in the atmosphere.
-
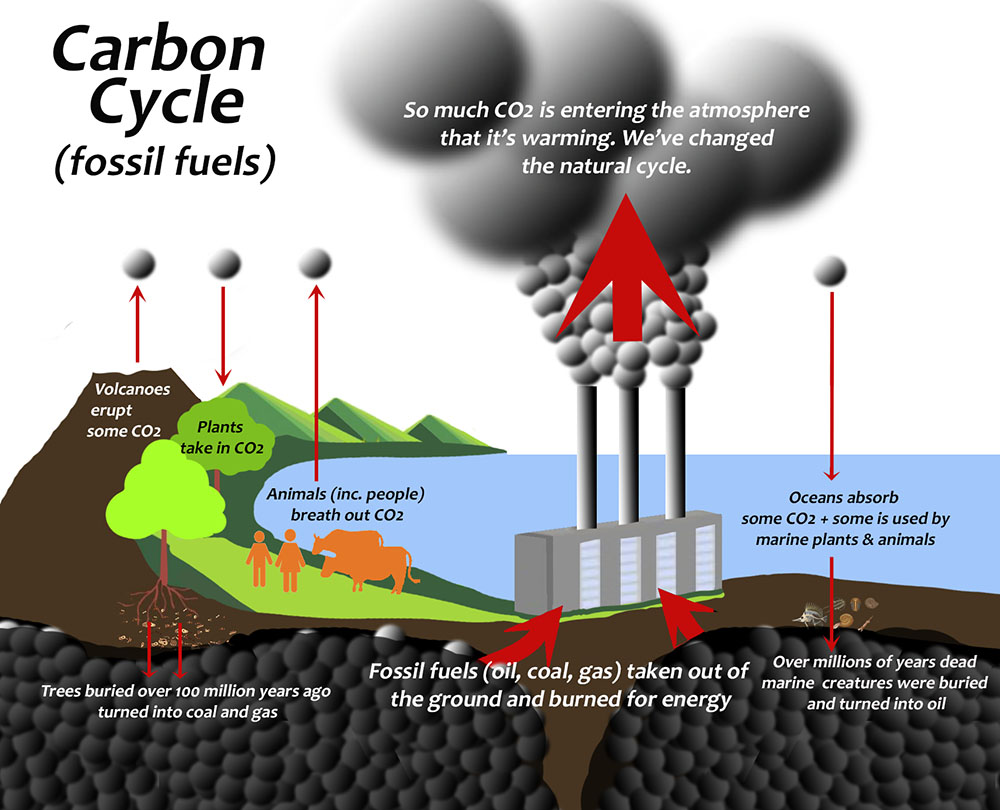 Fig. 2: The ‘fossil fuel’ part of the carbon cycle. Depending on conditions at the time, some dead plants and animals don’t decompose. See Figures 4 and 5 for more detailed explanation of how some become oil and coal.
Fig. 2: The ‘fossil fuel’ part of the carbon cycle. Depending on conditions at the time, some dead plants and animals don’t decompose. See Figures 4 and 5 for more detailed explanation of how some become oil and coal. NOTE: The processes described in Figures 2 and 3 (the ‘calcium carbonate’ part of the cycle – see below) work simultaneously.
-
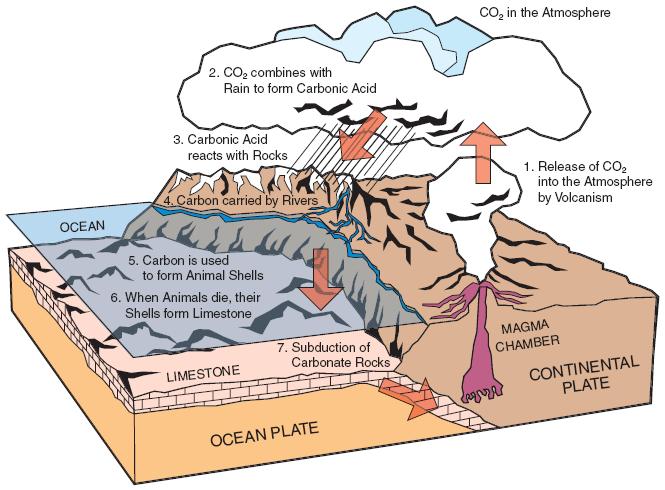
Fig. 3: The ‘calcium carbonate’ part of the carbon cycle. When marine animals including corals die, their skeletons and shells are buried and compressed. Some ultimately become the metamorphic rock limestone. This is moved deep underground (subducted) where tectonic plates grind against one another. Eventually, it becomes the main source of CO2 erupted by volcanoes. This mixes with rain to become slightly acidic, which in turn weathers rocks and carries carbon to the ocean where it is used by marine animals and corals, completing the cycle.
NOTE: The processes described in Figures 2 (the ‘fossil fuel’ part of the cycle – see above) and 3 work simultaneously.
Some 8% of carbon emissions comes from making cement, which is made from limestone. (Image: University of Illinois)
-
Carbon already in the atmosphere
(ppm = parts per million; Gt = one gigatonne or one billion tonnes)
- 2.13 Gt of carbon = 1 ppm currently in the atmosphere
- To convert carbon (C) to carbon dioxide (CO2), first divide the atomic mass of carbon (12) by the atomic mass of CO2 (44) = 3.67.
- Then multiply this by 2.13 Gt carbon: 3.67 x 2.13 = 7.8 Gt carbon dioxide
- So, 7.8Gt carbon dioxide = 1ppm of CO2 currently in the atmosphere
- As there is currently around 415ppm* of CO2 in the atmosphere, that’s 415 x 7.8 Gt = 3,373Gt CO2.
* The amount of CO2 in the atmosphere varies seasonally because plants accumulate carbon in the spring and summer and release some back to the air in autumn and winter. As the northern hemisphere has more land and plants, carbon dioxide levels go up in winter because plants become less productive. Annual measurements of carbon dioxide are an average of these ups and downs. On April 11, 2021, CO2 in the atmosphere peaked at 420ppm
Calculations for adding carbon to the atmosphere from emissionsEmissions are NOT the same as concentrations! This is because the ocean and biosphere absorb around 55% of emissions. Currently, about 45% stays in the atmosphere.- To calculate each additional ppm, divide 7.8 Gt / 0.45 = 17.3Gt
- So, it takes about 17.3Gt of CO2 emissions to add 1ppm to the atmosphere
But that number is changing because in the future, the oceans will not be able to absorb as much of this extra CO2 future, which means that fewer emissions will add more CO2 and thus more warming to the atmosphere.Moreover, “Additional ecosystem responses to warming not yet fully included in climate models, such as CO2 and CH4 [methane] fluxes from wetlands, permafrost thaw and wildfires, would further increase concentrations of these gases in the atmosphere (high confidence).” – IPCC 2021 p41.
-
During the 60-million year-long Carboniferous Period 386-299 million years ago, carbon dioxide in the atmosphere was ~800ppm. The climate was very warm and also wet (warmer air carries more moisture so there’s a lot more rain). Life has evolved to live in these conditions, so plants and animals thrived.The tectonic plates that would eventually form the super-continent Pangaea were colliding. Mountains (now the Appalachians in the US) were being pushed up, which forced the crust downward beneath soggy tropical wetland regions.Over millions of years, dead trees fell into the swampy ground. Here, they couldn’t be decomposed through normal processes that use oxygen. Instead, they turned into peat and eventually, coal.This unique combination of colliding tectonic plates, a warm wet climate, and trees that had slowly evolved to thrive in these conditions, is not likely to be repeated in Earth’s future. Even if similar circumstances arose in the future, it would take hundreds of millions of years for life to adapt and the cycle to begin (Fig. 4 below from Socratic.org).This is why coal is regarded as a non-renewable resource; it’s certainly not renewable in human terms.
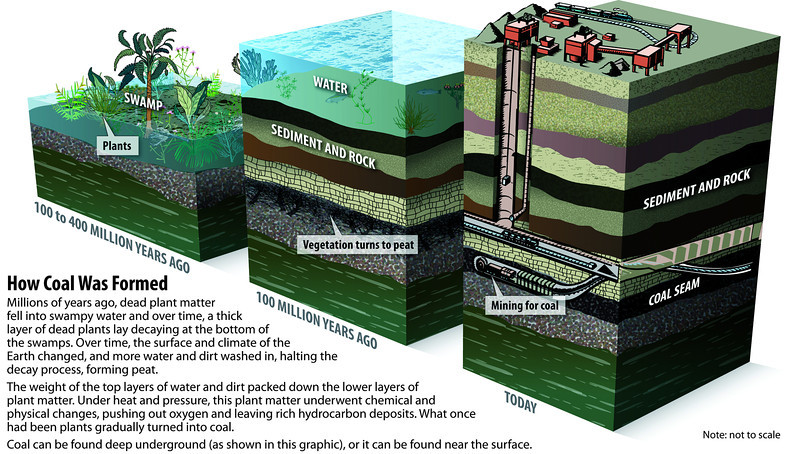
-
The conditions for oil formation were less unique than that of coal, however it is still regarded as a non-renewable fossil fuel because the process takes millions of years—far faster than oil is being extracted. Not all marine animals become oil, however. The vast bulk of them are are metamorphised into limestone. (Fig. 5 below from Socratic.org).
Today, limestone is used to make cement, which is one of the major contributor to greenhouse gasses (see the section on ‘limestone’ above).
-
Two reasons:1. Volcanoes erupt CO2 along with other chemicals and rock that’s been melted deep underground. Much of this rock formed hundreds of millions of years earlier and is recycled by subduction (plate tectonics). Because rock is melted by heat, not combustion (burning), no oxygen is used, so oxygen levels should have stayed the same if volcanoes are to blame. In fact, oxygen levels in the atmosphere are dropping:

This graph shows the decline in oxygen measured at Cape Grim, Tasmania. The location was selected to measure Earth’s atmosphere in 1976 because winds from Antarctica and the Indian Ocean hit no significant land masses in the way. The ups and downs in the graph are because there is a natural annual summer/winter ‘cycle’ in the atmosphere.
2. The CO2 from volcanoes has a different isotopic ratio than the CO2 from burning fossil fuels. It has long been known that Carbon comes in three forms (isotopes): 12C (the most common), 13C and 14C. Professor Richard Alley explains the process in the 2-minute video below.“CO2 produced from burning fossil fuels or burning forests has quite a different isotopic composition from CO2 in the atmosphere. This is because plants have a preference for the lighter isotopes (12C versus 13C); thus they have lower 13C/12C ratios. Since fossil fuels are ultimately derived from ancient plants, plants and fossil fuels all have roughly the same 13C/12C ratio—about 2% lower than that of the atmosphere. As CO2 from these materials is released into, and mixes with, the atmosphere, the average 13C/12C ratio of the atmosphere decreases.” – Eric Steig, isotope geochemist, University of Washington, Seattle.For more technical information see: Stuiver et al (1984): 13C/12C ratios and the transfer of biospheric carbon to the atmosphere, Journal of Geophysical Research: Atmospheres 89/D7, 11731-1748.
-
This is often called the CO2 ‘fertilisation effect’
The speeding-up of photosynthesis—known as ‘CO2 fertilisation’—is well-known to be an important outcome of higher CO2 concentrations, along with increased water use efficiency. This is because as CO2 in the atmosphere increases, plants don’t lose as much water through their leaves because the number of their stoma decreases. So it might seem that drier conditions shouldn’t have such a large impact.
However, there are 5 things wrong with this assumption:
1. The nutritional values of plants is declining as more carbon dioxide levels go up. So animals and people will need to eat larger quantities (more calories) in order to get the same nutritional benefit as before. Physicist and science educator Dr. Derek Muller explains this in the video below.2. Orchard crops (apples, pears, Kiwi fruits, avocadoes, grapes, cherries etc) can grow only within a certain optimal temperature range suited to each species. It can take several years for orchards to reach maturity. An orchard planted today may not survive a future climate by the time it reaches maturity. Or it may be less productive for fewer year and more prone to pests and diseases, making them too expensive to maintain. For a more detailed explanation see the NZ Science Blog on this subject.
3. Diseases thrive in a warming climate, and this includes plant diseases. An orchard crop may be productive for fewer year and more prone to pests and diseases, making them too expensive to maintain. For a more detailed explanation see the NZ Science Blog on this subject.
“As we have seen, pathogens tend to migrate to follow suitable climates, as long as their hosts are present. This means that as humans respond to climate change with altered agricultural practice, crop diseases are likely to keep pace.” – Dr Helen Fones, 2020
4. Rapid plant growth is leading to increased warming and droughts: As the climate warms, warmer spring temperatures are also arriving earlier. In the Arctic and sub-Arctic, plant leaves are coming out sooner each year. This early ‘leaf-out’ is triggering an array of feedback effects including increased surface warming in the Northern Hemisphere Arctic:
“We identify warming hotspots in the Canadian Arctic Archipelago (~0.7 °C), east and west edges of Siberia (~0.4 °C) and southeastern Tibetan Plateau (~0.3 °C). …With continued warming, positive feedbacks between climate and leaf phenology are likely to amplify warming in the northern high latitudes.” – Xu et al, 2020
The Arctic is not alone in experiencing the not-so beneficial impacts of rapid springtime plant growth. Early and rapid spring growth across Europe in 2018 led to plants sucking up large quantities of water from the soil. By summer, the ‘legacy effect’ amplified summer drought conditions in areas that already were heat and/or water stressed.
“Spring conditions led to an enhancement of photosynthesis early on in the growing season, but at the cost of strong soil-water depletion. In the crop-dominated areas in central Europe, increased growth in spring made ecosystems more vulnerable to drought in summer.” – Dr Anna Bastos, 2020
The assumption that tropical forests across Africa and the Amazon will continue to absorb endless quantities of CO2 also has recently been brought into question. While forests that have not yet been burned for agriculture continue to grow larger and absorb CO2…
“Across Africa and the Amazon…higher temperatures and stronger drought conditions are slowing plant growth—and killing trees.” – Hubai et al, 2020
5. The hotter it gets, the more CO2 plants release back into the atmosphere.
“Currently, around 25 per cent of carbon emissions from the use of fossil fuels is being taken up and stored by plants, which is good, as it helps reduce the concentration of greenhouse gases in the atmosphere.Our work suggests that this positive contribution of plants may decline in the future as they begin to respire more as the world warms.” – Prof. Atkin, ANU
“Photosynthesis peak at about 18C in many of the world’s leafy areas, then went into decline. However, respiration kept increasing, so plants were breathing out greenhouse gas faster, while taking progressively less in. With enough heat, they’d flip from sinks to sources.” – Gibson, Stuff article on the above research
-
Permafrost is a combination of soil, sediment, and the remains of dead plants and animals that stay at or below 0°C for at least two years. Unlike ice, it doesn’t ‘melt’ once temperatures rise above 0°C. Permafrost falls apart, and the organic material decomposes, just as frozen meat or vegetables left outside a freezer will decompose if not eaten.
Permafrost can be as thin as <1m and as thick as >1,000m. It covers approximately 22.79 million km² (about 24% of the exposed land surface) of the Northern Hemisphere.
If decomposition occurs in an environment where there’s oxygen, then carbon dioxide gas is released. Some of this may be used by plants. If the environment is anaerobic (lacks oxygen), methane is released; this goes directly into the atmosphere (Fig. 5 below. & Video 2).
Fig. 5: “Across nine million square miles at the top of the planet, climate change is writing a new chapter. Arctic permafrost isn’t thawing gradually, as scientists once predicted. Geologically speaking, it’s thawing almost overnight. As soils like the ones at Duvanny Yar soften and slump, they’re releasing vestiges of ancient life—and masses of carbon—that have been locked in frozen dirt for millennia.” – Craig Welsh, September 2019 issue of National Geographic magazine. Photo: Katie Orlinsky (see her entire photo essay on melting permafrost).
Melting permafrost is the result of a dangerous natural feedback effect of climate change, whereby anthropogenic forcing is triggering natural forcing. While we can control the amount of fossil fuels we burn, we cannot control how nature responds.
The IPCC has determined that the maximum ‘safe’ temperature Earth can reach is less than 2°C on average, and preferably no more than 1.5°C for disastrous impacts to be avoided. However, the Arctic has already warmed much faster than anywhere else on Earth; it’s average temperature is now 4°C above what it was in 1960.
In NOAA’s 2019 report card, it’s estimated that melting permafrost is contributing 600 million metric tonnes of net carbon per year into Earth’s atmosphere (Video 3: NOAA summary of the State of the Arctic 2019). In 2021, the remaining carbon budget to have an 85% chance of staying under 1.5°C, is around 280 billion tonnes.
“By 2100, near-surface permafrost area will decrease by 2-66% for RCP2.6 and 30–99% for RCP8.5. This could release 10s to 100s of gigatonnes of carbon as CO2 and methane to the atmosphere for RCP8.5.” – IPCC, 2019
In sum, every bit of permafrost that melts reduces our remaining carbon budget to stay under 1.5°C.
-
Earth has periodically been hit by asteroids, comets, and other space debris large enough to instantly change the climate. The best-known event was the Cretaceous–Paleogene (K–Pg) extinction event ~65 million years ago when an asteroid impact blew dust, soil, and rocks not only into the atmosphere but also out into space, where it fell back into the upper atmosphere, creating a dust shroud for weeks to months. This stopped sunlight from reaching the surface, which led to a cool ‘impact winter’ for some years.
Worse, the asteroid slammed into rocks rich in carbonates, that is, they were full of carbon. This, along with global-scale wildfires that also released huge quantities of CO2 into the atmosphere from burning trees, led to a rapid global warming of ~5°C soon after the relatively brief ‘impact winter’ cooling period.
- Evidence of the impact event can be seen in a layer of iridium (dust from the comet) in the Waipara River, North Canterbury.
- There is also evidence that large scale volcanic eruptions in what is now Northern India also contributed to the warming.
- Dust from an extraterrestrial impact event near (but not into) Earth ~466 million years has been implicated in an an ‘impact winter’ that led to an Ice Age.
- The Eocene–Oligocene extinction event 35 million years ago may have been triggered in part by up to five impact events including at Popigai, Siberia, and Chesapeake Bay, US.
-
Relative to the amount of air in the atmosphere, it takes only a small change in the amount of carbon dioxide in the atmosphere to switch Earth’s climate from warm to cold.
In the year 1850 (before humans began burning large amounts of fossil fuels), the amount of carbon dioxide in the atmosphere was 280 parts per million (ppm) and the average temperature was 13.7°C.
Today, with around 420ppm, the average temperature is around 15°C.
Other greenhouse gases have added to this temperature change, but carbon is the gas that’s had the most impact. To find out more about the other gases, see Greenhouse gases and how they work (this website).
-
If more solar energy escapes than arrives, the planet cools. Conversely, if less energy escapes than gets in, the planet warms. Some of these forcings are due to natural events, so are called ‘natural’ forcings, some of which are cyclic. Others are caused by human activities. These are called ‘anthropogenic’ forcings:
Forcings:
- Greenhouse gases and aerosols
- Land use changes destroying biodiversity (anthropogenic)
- The Milankovich Cycle (natural): how Earth orbits the Sun
- Sunspots and solar activity (natural): variations in solar energy
- Plate tectonics (natural): the position of continents
- Ocean currents (natural): distributing heat and nutrients
- Iron flux (natural): fertilising life in the oceans
- Rocks from space (natural): not often, but dramatic!
Click here to learn about the main forcings and how they work (links to a page on this site).
-
Around 2.4 – 2 billion years ago during the Paleoproterozoic era, the earliest life forms were anaerobic cyanobacteria, which produced oxygen as a waste product. There was so many of them producing so much oxygen that that they changed the chemistry of the atmosphere…and poisoned themselves in the process.This is known as the Great Oxidation Event, also called the Great Oxygenation Event, the Oxygen Crisis and the Oxygen Catastrophe. While almost all of them died out, some were engulfed by eukaryotes to become endosymbiotic cyanobacteria. Over hundreds of millions of years, they evolved into chloroplasts: the green parts inside of plants that we see today, responsible for photosynthesis. They still produce the oxygen we need to survive, and now play an essential role in the carbon cycle.
-
The amount being lost is tiny—about nineteen O2 molecules out of every 1 million O2 molecules in the atmosphere each year. This small loss provides the chemical evidence that points the finger at humans burning fossil fuels, but it doesn’t affect our ability to breathe.
-
- 2023: Smith; State dependence of CO2 forcing and its implications for climate sensitivity. Science 382|6674 pp1051-1056
- 2023: Yu et al; Millennial atmospheric CO2 changes linked to ocean ventilation modes over past 150,000 years, Nature Geoscience article (Open access).
- IPCC (undated): The Carbon Cycle
- Ministry for the Environment: New Zealand Emissions Trading Scheme
- Ministry for the Environment: Climate Change Response (Zero Carbon) Amendment Act
- Ministry for the Environment: New Zealand’s Greenhouse Gas Inventory
- Carbon Brief Explainer: How the rise and fall of CO2 levels influenced the ice ages
- Carbon Brief: Why scientists think 100% of global warming is due to humans
- Daily Carbon Tracker
- Science Direct: The Law of Conservation of Energy
- NSIDC: State of the Cryosphere: Permafrost and Frozen Land
- Scripps Institute of Oceanography: O2 Program
- NOAA: Global Monitoring Laboratory – Carbon tracker Moana Loa
- NOAA: Climate forcing
- University of Illinois: the geological carbon cycle (limestone and plate tectonics)
- 2021: Gibson; Earth’s ‘lungs’ could start deteriorating in as little as two decades – Stuff article
- 2020: Grass and the science of CO2 Radio NZ Podcast
- 2020: Soil carbon unearthed Nature Geoscience (editorial) 13/ 523
- 2020: Hubau et al; Asynchronous carbon sink saturation in African and Amazonian tropical forests, Nature 579, pp80–87
- 2020: Bastos et al; Direct and seasonal legacy effects of the 2018 heat wave and drought on European ecosystem productivity, Science Advances 6/24
- Carbon Brief article on the above: Fones; Guest post: How climate change could accelerate the threat of crop diseases
- 2020: Du et al; Global patterns of terrestrial nitrogen and phosphorus limitation, Nature Geoscience 13, pp221–226
- 2020: Xu et al; Earlier leaf-out warms air in the north; Nature Climate Change 10, pp370–375:
- 2019: NOAA Richter-Menge et al; Arctic Report Card 2019
- 2019: IPCC: Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories
- 2019: IPCC: Special Report on the Ocean and Cryosphere in a Changing Climate; Chapter 3: Polar Regions (Section 3.4: Arctic Snow, Freshwater Ice and Permafrost: Changes, Consequences and Impacts)
- 2019: Solly; Carbon Dioxide Levels Reach Highest Point in Human History Smithsonian magazine
- 2019: National Geographic: The Arctic Is Heating Up September 2019 special issue
- 2018: IPCC: Chapter 10 Industry in Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change
- 2018: Santhanam et al; Greenhouse Gas Sensors Fabricated with New Materials for Climatic Usage: A Review Chemical Engineering 2(3) 38
- 2018: Carbon Brief Q&A: Why cement emissions matter for climate change
- 2017: Huntingford et al; Implications of improved representations of plant respiration in a changing climate, Nature Communications 8/1602
- 2016: Nelson et al; Delayed fungal evolution did not cause the Paleozoic peak in coal production; PNAS 13 (9) 2442-2447
- 2017: Andrew; Global CO2 emissions from cement production, Earth Syst. Sci. Data, 10, 195–217, 201
- 2013: IPPC: Chapter 8: Anthropogenic and Natural Radiative Forcing in: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change
- 2012: Shakun et al; Global warming preceded by increasing carbon dioxide concentrations during the last deglaciation Nature 484 49-54
- 2001: Etheridge et al; Law Dome Atmospheric CO2 Data, IGBP PAGES/World Data Center for Paleoclimatology Data Contribution Series #2001-083. NOAA/NGDC Paleoclimatology Program, Boulder CO, USA.
- 1984 Stuiver et al; 13C/12C ratios and the transfer of biospheric carbon to the atmosphere. Journal of Geophysical Research: Atmospheres 89/D7, 11731-1748.
- 1856: Foote; Circumstances affecting the heat of the sun’s rays American Journal of Science and Arts; Vol. XXII 377-383
-
- 2023: Smith; State dependence of CO2 forcing and its implications for climate sensitivity. Science 382|6674 pp1051-1056
- IPCC (undated): The Carbon Cycle
- 2023: Yu et al; Millennial atmospheric CO2 changes linked to ocean ventilation modes over past 150,000 years, Nature Geoscience article (Open access).
- Ministry for the Environment: New Zealand Emissions Trading Scheme
- Ministry for the Environment: Climate Change Response (Zero Carbon) Amendment Act
- Ministry for the Environment: New Zealand’s Greenhouse Gas Inventory
- Carbon Brief Explainer: How the rise and fall of CO2 levels influenced the ice ages
- Carbon Brief: Why scientists think 100% of global warming is due to humans
- Daily Carbon Tracker
- Science Direct: The Law of Conservation of Energy
- NSIDC: State of the Cryosphere: Permafrost and Frozen Land
- Scripps Institute of Oceanography: O2 Program
- NOAA: Global Monitoring Laboratory – Carbon tracker Moana Loa
- NOAA: Climate forcing
- University of Illinois: the geological carbon cycle (limestone and plate tectonics)
- 2021: Gibson; Earth’s ‘lungs’ could start deteriorating in as little as two decades – Stuff article
- 2020: Grass and the science of CO2 Radio NZ Podcast
- 2020: Soil carbon unearthed Nature Geoscience (editorial) 13/ 523
- 2020: Hubau et al; Asynchronous carbon sink saturation in African and Amazonian tropical forests, Nature 579, pp80–87
- 2020: Bastos et al; Direct and seasonal legacy effects of the 2018 heat wave and drought on European ecosystem productivity, Science Advances 6/24
- Carbon Brief article on the above: Fones; Guest post: How climate change could accelerate the threat of crop diseases
- 2020: Du et al; Global patterns of terrestrial nitrogen and phosphorus limitation, Nature Geoscience 13, pp221–226
- 2020: Xu et al; Earlier leaf-out warms air in the north; Nature Climate Change 10, pp370–375:
- 2019: NOAA Richter-Menge et al; Arctic Report Card 2019
- 2019: IPCC: Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories
- 2019: IPCC: Special Report on the Ocean and Cryosphere in a Changing Climate; Chapter 3: Polar Regions (Section 3.4: Arctic Snow, Freshwater Ice and Permafrost: Changes, Consequences and Impacts)
- 2019: Solly; Carbon Dioxide Levels Reach Highest Point in Human History Smithsonian magazine
- 2019: National Geographic: The Arctic Is Heating Up September 2019 special issue
- 2018: IPCC: Chapter 10 Industry in Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change
- 2018: Santhanam et al; Greenhouse Gas Sensors Fabricated with New Materials for Climatic Usage: A Review Chemical Engineering 2(3) 38
- 2018: Carbon Brief Q&A: Why cement emissions matter for climate change
- 2017: Huntingford et al; Implications of improved representations of plant respiration in a changing climate, Nature Communications 8/1602
- 2016: Nelson et al; Delayed fungal evolution did not cause the Paleozoic peak in coal production; PNAS 13 (9) 2442-2447
- 2017: Andrew; Global CO2 emissions from cement production, Earth Syst. Sci. Data, 10, 195–217, 201
- 2013: IPPC: Chapter 8: Anthropogenic and Natural Radiative Forcing in: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change
- 2012: Shakun et al; Global warming preceded by increasing carbon dioxide concentrations during the last deglaciation Nature 484 49-54
- 2001: Etheridge et al; Law Dome Atmospheric CO2 Data, IGBP PAGES/World Data Center for Paleoclimatology Data Contribution Series #2001-083. NOAA/NGDC Paleoclimatology Program, Boulder CO, USA.
- 1984 Stuiver et al; 13C/12C ratios and the transfer of biospheric carbon to the atmosphere. Journal of Geophysical Research: Atmospheres 89/D7, 11731-1748.
- 1856: Foote; Circumstances affecting the heat of the sun’s rays American Journal of Science and Arts; Vol. XXII 377-383